Role of SIRT7 in FLT3 ITD mediated aging and differentiation of hematopoietic stem cells
Oncogenic FLT3 ITD controls differentiation of HSC via Sirt7
Graphic: Jörg P. MüllerResearch topic(s)
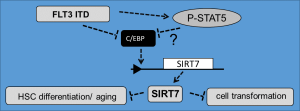
SIRT7 has been identified as factor regulating hematopoietic stem cell (HSC) dormancy and aging. Reduction of SIRT7 results in initiation of HSC differentiation. Our studies revealed reduced SIRT7 levels in cells expressing the oncogenic FLT3 ITD receptor tyrosine kinase. Suppression of SIRT7 was controlled by the aberrant FLT3 ITD kinase activity. Reconstitution of SIRT7 in FLT3 ITD cells suppressed cell differentiation, gene inactivation stimulated it. Important, AML patients with low SIRT7 have poor prognosis demonstrating clinical relevance of this protein for patient survival (Kaiser et al., Leukemia 2020External link).
By using in vitro and in vivo systems, we are going to characterize the mechanism, how FLT3 ITD controls HSC aging and differentiation via SIRT7 in leukemic cell systems. In vivo reconstitution of transduced BM cells with altered SIRT7 activities into FLT3 ITD mice provide insight into the mechanism, how SIRT7 affects HSC aging and development of the myeloproliferative disease and clonal expansion. Description of underlying signaling pathways mediating HSC aging and differentiation provide a critical insight into the pathogenesis of hematological and will open new ways to control leukemic development.
Methods
In vitro techniques:
- Molecular biological manipulation of cell lines
- Transient and virus mediated suppression and overexpression of genes
- CRISPR/Cas-mediated inactivation of genes, (gene specific, genome wide)
- CRISPRa, CRISPRi applications
- Imaging techniques for spatial and temporal protein localization and protein complex formation
In vivo techniques:
- Mouse models overexpressing oncogenes
- Syngenic mouse models
- Bone marrow transduction and transplantation
- CRE-mediated tissue specific inactivation of genes
- Pharmacological applications on mice
Selected publications
Bär I, Ast V, Meyer D, König R, Rauner M, Hofbauer LC, Müller JP. (2020) Aberrant Bone Homeostasis in AML Is Associated with Activated Oncogenic FLT3-Dependent Cytokine NetworksExternal link. Cells, 9(11):2443
Müller JP, Schmidt-Arras D. (2020) Novel Approaches to Target Mutant FLT3 Leukaemia.External link Cancers (Basel), 12(10)
Kaiser A, Schmidt M, Huber O, Frietsch JJ, Scholl S, Heidel FH, Hochhaus A, Müller JP, Ernst T. (2020) SIRT7: an influence factor in healthy aging and the development of age-dependent myeloid stem-cell disorders.External link Leukemia, 34(8):2206-2216
Kellner F, Keil A, Schindler K, Tschongov T, Hünninger K, Loercher H, Rhein P, Böhmer SA, Böhmer FD, Müller JP. (2020) Wild-type FLT3 and FLT3 ITD exhibit similar ligand-induced internalization characteristics.External link J Cell Mol Med, 24(8):4668-4676
Kresinsky A, Schnöder TM, Jacobsen ID, Rauner M, Hofbauer LC, Ast V, König R, Hoffmann B, Svensson CM, Figge MT, Hilger I, Heidel FH, Böhmer FD, Müller JP. (2019) Lack of CD45 in FLT3-ITD mice results in a myeloproliferative phenotype, cortical porosity, and ectopic bone formationExternal link. Oncogene, 38(24):4773-4787